

Acne Vulgaris Patient ProfilesGET TO KNOW 2 DIFFERENT PATIENT TYPES.
Read Patient ProfilesPROVEN EFFICACY IN TREATING
ACNE VULGARIS IN
2,000+
PATIENTS ENROLLED IN
2 AKLIEF CREAM CLINICAL TRIALS2
IMPROVEMENT IN BOTH FACIAL AND TRUNCAL ACNE.2
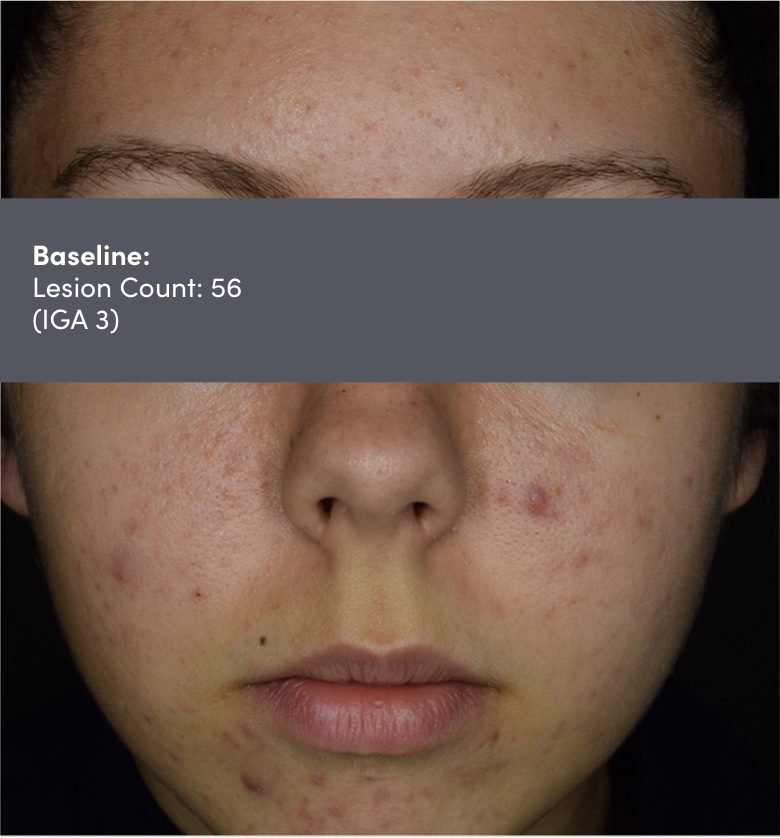
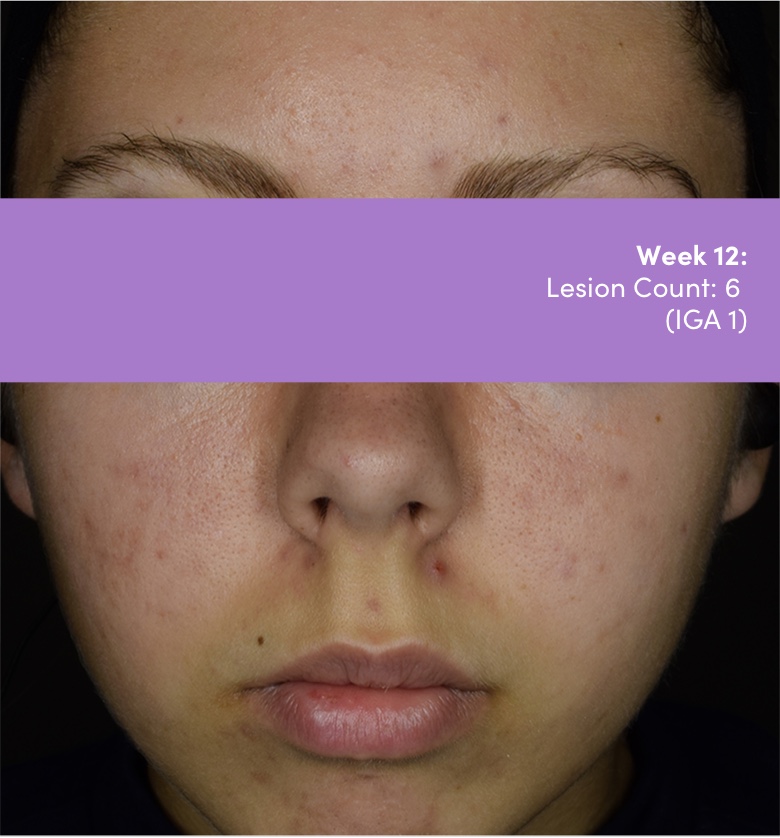
IGA=Investigator's Global Assessment.
because clearer skin matters.
PIVOTAL STUDIES 1 & 2
These studies were the first large-scale, randomized, Phase 3 clinical trials to simultaneously evaluate a topical therapy for the treatment of both moderate facial and truncal acne vulgaris.1,2
Study 18251: Double-blind, randomized, vehicle-controlled safety and efficacy 12-week trial of trifarotene cream vs vehicle cream.
Subject: 8511-004 | Age: 16 | Gender: Female



