AKLIEF® PIVOTAL Studies 1 & 2
First large scale trials to investigate truncal acne.1,2
PIVOTAL Studies 1 & 2 were the first large-scale, randomized, Phase 3 clinical trials to simultaneously evaluate a topical therapy for the treatment of both moderate facial and truncal acne vulgaris.1
STUDY DESIGN
2,420
PATIENTS RANDOMIZED 1:1
From sites across US, Canada, Europe, and Russia;
Mean age: 19 years (9-58 years); AKLIEF Cream once daily1
AKLIEF Cream
n=1,214
VEHICLE
n=1,206

PIVOTAL Studies 1 & 2
PRIMARY ENDPOINTS:
FACE1
- Success rate: Percentage of subjects with IGA* of clear (0) or almost clear (1) and at least a 2-grade improvement from baseline to Week 12
- Absolute change in facial inflammatory and non-inflammatory lesion count from baseline to Week 12
SAFETY ENDPOINTS1
- Incidence of adverse events
- Local tolerability (erythema, scaling, dryness, stinging/burning)
*The definitions of severity for the 5-point IGA and PGA scales were the same: 0 (clear), 1 (almost clear), 2 (mild), 3 (moderate), and 4 (severe).
IGA=Investigator’s Global Assessment.
PIVOTAL Studies 1 & 2
SECONDARY ENDPOINTS:
TRUNK1
- Success rate: Percentage of subjects with PGA* of clear (0) or almost clear (1) and at least a 2-grade improvement from baseline to Week 12
- Absolute change in truncal inflammatory and
non-inflammatory lesion count from baseline to Week 12
*The definitions of severity for the 5-point IGA and PGA scales were the same: 0 (clear), 1 (almost clear), 2 (mild), 3 (moderate), and 4 (severe).
PGA=Physician Global Assessment.
AKLIEF Cream
THE FIRST TOPICAL ACNE TREATMENT WITH PROVEN RESULTS ON THE FACE AND TRUNK.2
SIGNIFICANT IMPROVEMENT IN TOTAL FACIAL LESION COUNT AT WEEK 12 VS VEHICLE.3
50.8%
FEWER TOTAL LESIONS AT
WEEK 12 VS BASELINE
VS 38.7% REDUCTION WITH VEHICLE
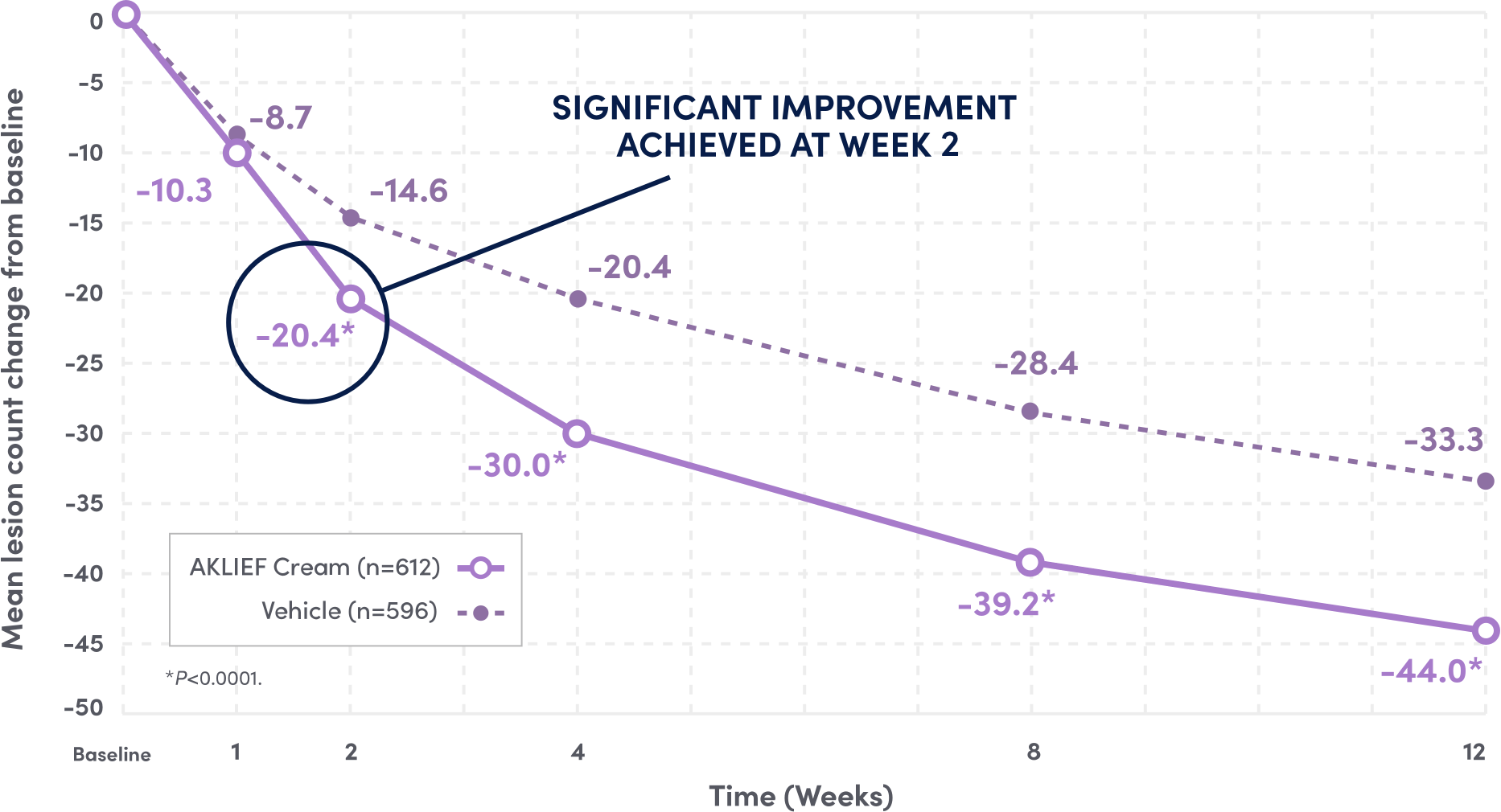
PIVOTAL Primary endpoints: 1) Absolute change in facial inflammatory and non-inflammatory lesion counts from baseline to Week 12; 2) Investigator's Global Assessment (IGA) success rate on the face.1
SIGNIFICANT REDUCTION IN FACIAL INFLAMMATORY LESIONS AT WEEK 12 VS VEHICLE.1
61.3%
REDUCTION IN INFLAMMATORY LESIONS VS BASELINE AT 12 WEEKS.1
VS 51.2% REDUCTION WITH VEHICLE; P<0.001.
PIVOTAL Primary endpoints: 1) Absolute change in facial inflammatory and non-inflammatory lesion counts from baseline to Week 12; 2) Investigator’s Global Assessment (IGA) success rate on the face.1
AKLIEF Cream
THE FIRST TOPICAL ACNE TREATMENT WITH PROVEN RESULTS ON THE FACE AND TRUNK.2
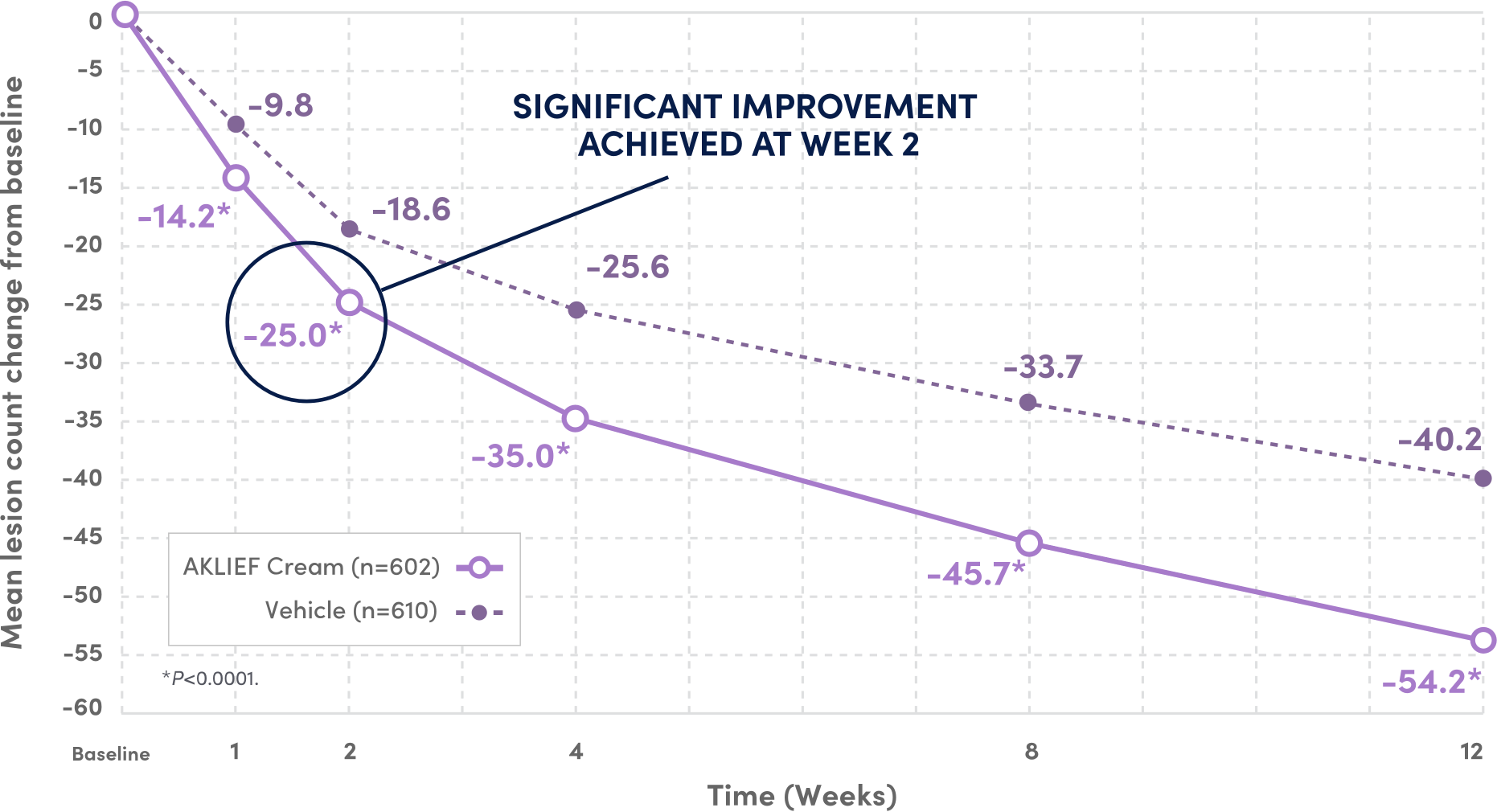
SIGNIFICANT IMPROVEMENT IN TOTAL TRUNCAL LESION COUNT AT WEEK 12 VS VEHICLE.3
52.3%
FEWER TOTAL LESIONS AT WEEK 12 VS BASELINE
VS 43.8% REDUCTION WITH VEHICLE3
PIVOTAL Secondary endpoints: 1) Absolute change in truncal inflammatory and non-inflammatory lesion counts from baseline at Week 12; 2) Physician's Global Assessment (PGA) success rate on the trunk.1
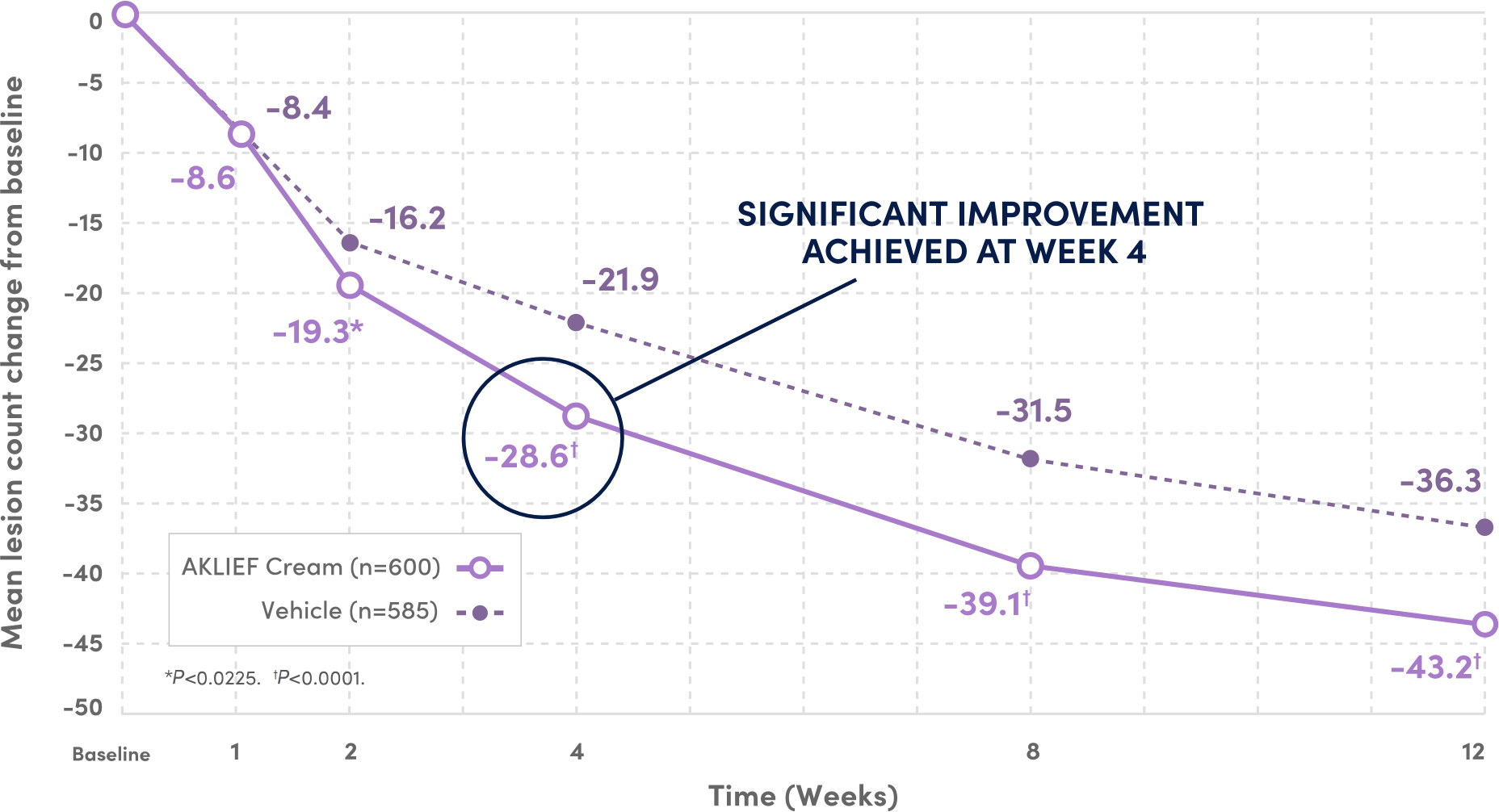
SIGNIFICANT IMPROVEMENT IN TOTAL TRUNCAL LESION COUNT AT WEEK 12 VS VEHICLE.4
59.9%
FEWER TOTAL LESIONS AT WEEK 12 VS BASELINE
VS 46.6% REDUCTION WITH VEHICLE4
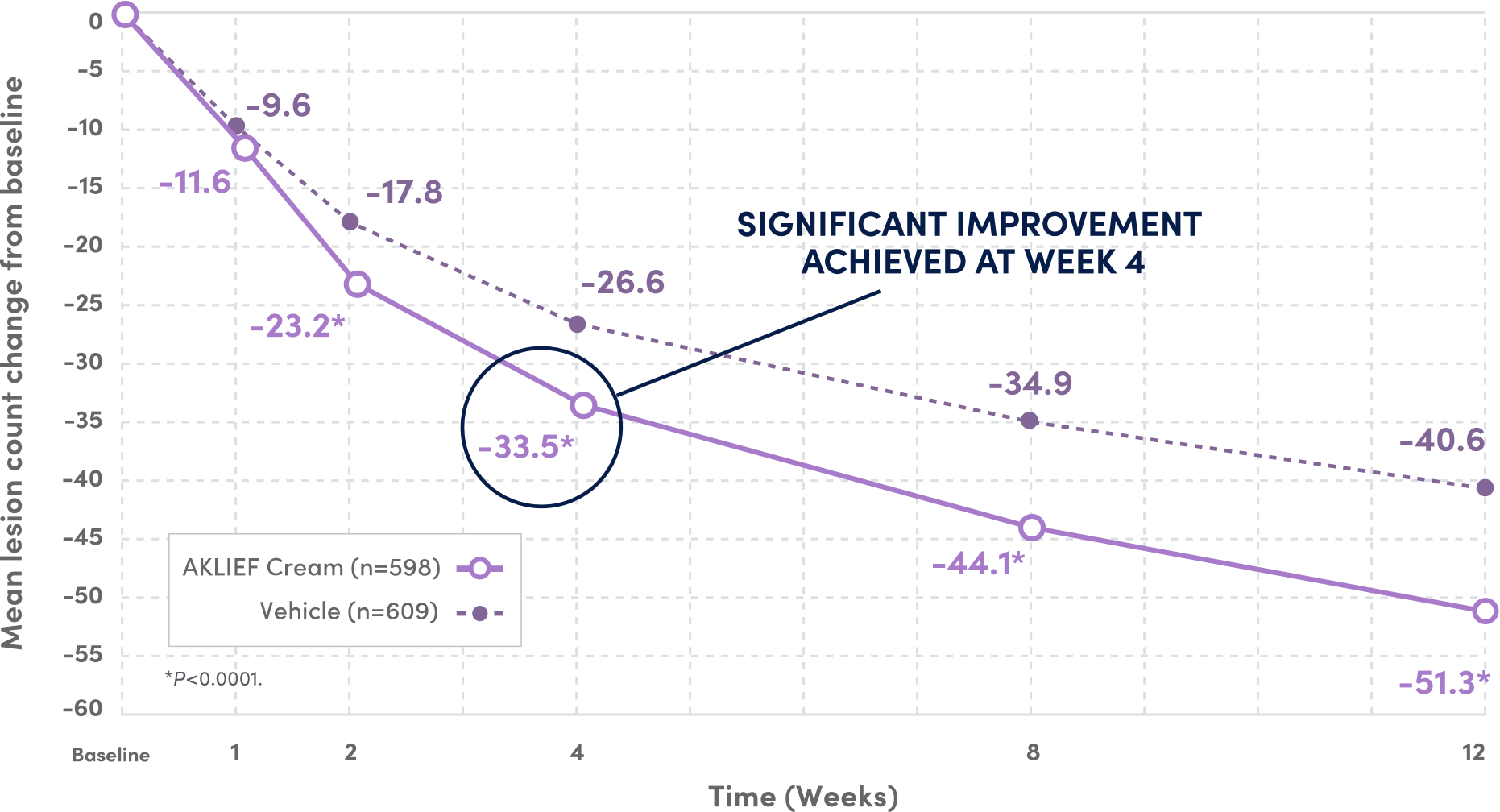
PIVOTAL Secondary endpoints: 1) Absolute change in truncal inflammatory and non-inflammatory lesion counts from baseline at Week 12; 2) Physician's Global Assessment (PGA) success rate on the trunk.
SAFE AND WELL-TOLERATED ON BOTH THE FACE AND TRUNK AT WEEK 12.1
PIVOTAL STUDY 1: TOLERABILITY SIGNS AND SYMPTOMS
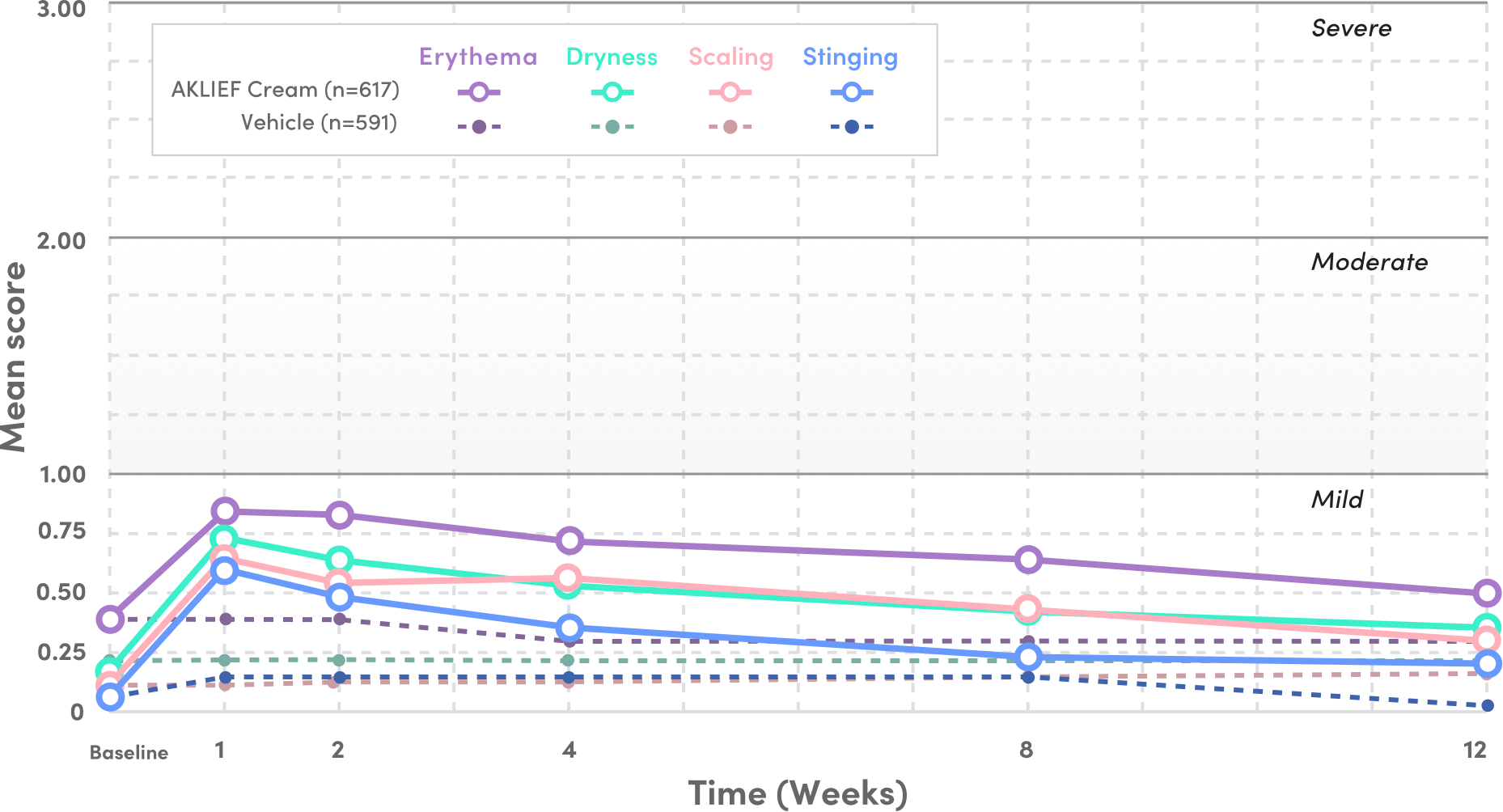
- Mean tolerability scores for erythema, scaling, dryness, and stinging/burning peaked at Week 1 on the face and decreased thereafter1
- Local tolerability signs and symptoms were evaluated on a 4-point severity scale: 0 (none), 1 (mild), 2 (moderate), and 3 (severe) from baseline to Week 12—all scores stayed below 1 through Week 121
SAFE AND WELL-TOLERATED ON BOTH THE FACE AND TRUNK.1
PIVOTAL STUDY 1: TOLERABILITY SIGNS AND SYMPTOMS
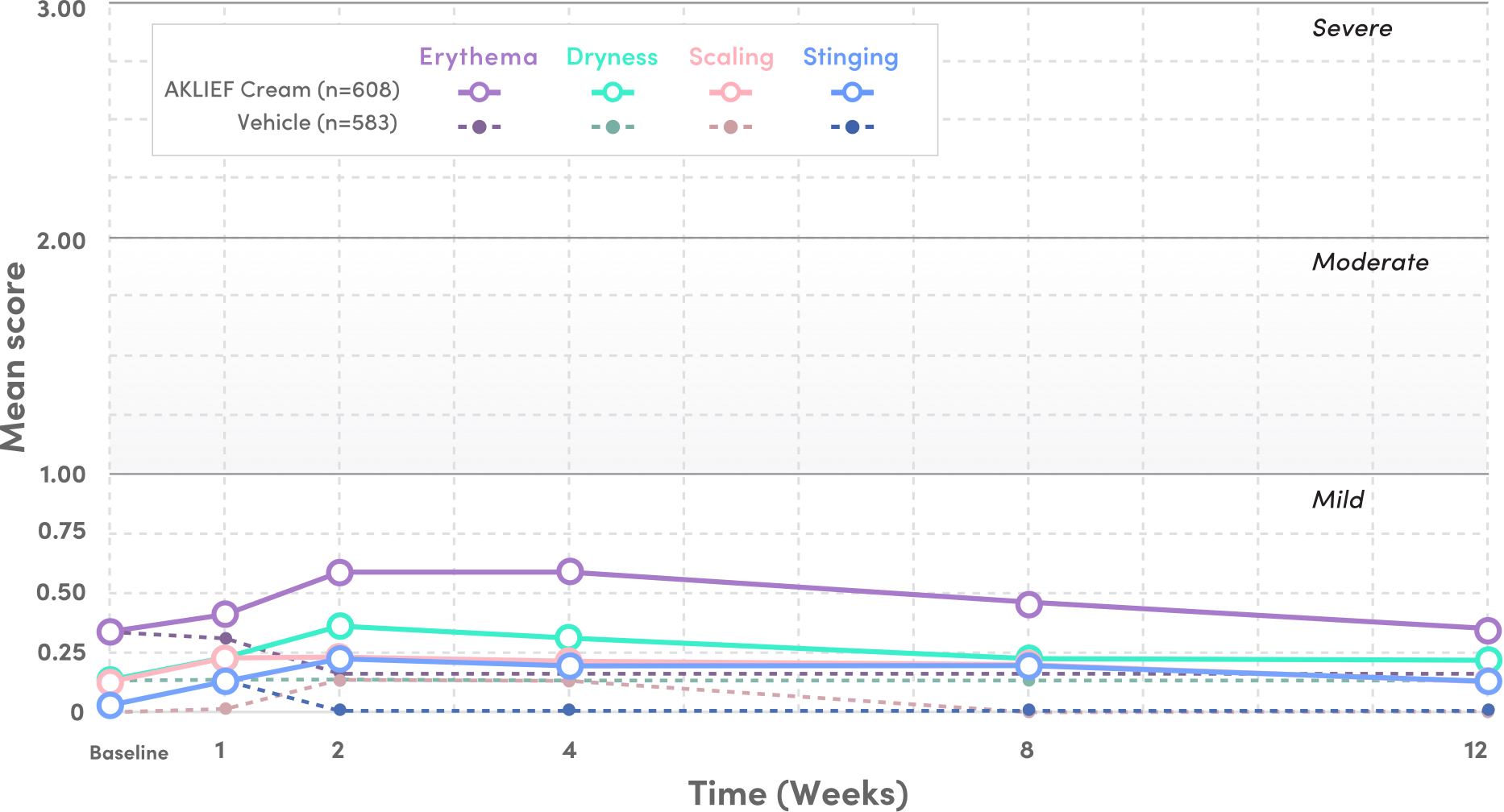
- Mean tolerability scores for erythema, scaling, dryness, and stinging/burning peaked at Week 1 on the face and decreased thereafter1
- Local tolerability signs and symptoms were evaluated on a 4-point severity scale: 0 (none), 1 (mild), 2 (moderate), and 3 (severe) from baseline to Week 12—all scores stayed below 1 through Week 121
In a long-term safety study,
WELL-TOLERATED THROUGHOUT THE STUDY AT 52 WEEKS.5
SATISFY Study: A multicenter, open-label study of 453 patients with moderate facial and truncal acne assessing the long-term safety (primary endpoint), efficacy, and quality of life (secondary endpoints) of AKLIEF Cream over 52 weeks.
PIVOTAL STUDY 1: TOLERABILITY SIGNS AND SYMPTOMS
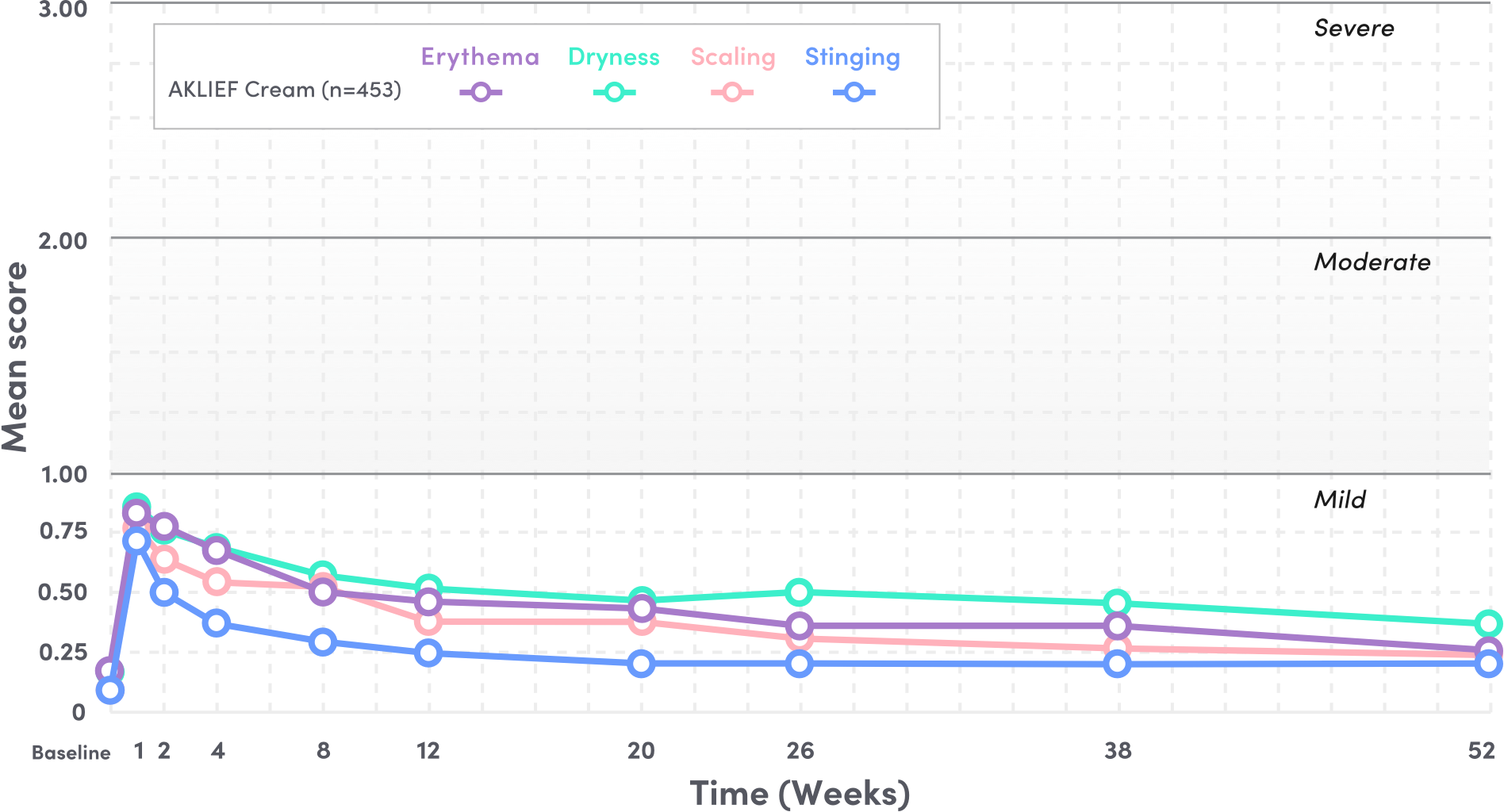
- Mean tolerability scores for erythema, scaling, dryness, and stinging/burning peaked at Week 1 on the face and decreased thereafter5
- Local tolerability signs and symptoms were evaluated on a 4-point severity scale: 0 (none), 1 (mild), 2 (moderate), and 3 (severe) from baseline to Week 52—all scores stayed below 1 through Week 525
In a long-term safety study,
WELL-TOLERATED THROUGHOUT THE STUDY AT 52 WEEKS.5
SATISFY Study: A multicenter, open-label study of 453 patients with moderate facial and truncal acne assessing the long-term safety (primary endpoint), efficacy, and quality of life (secondary endpoints) of AKLIEF Cream over 52 weeks.
PIVOTAL STUDY 1: TOLERABILITY SIGNS AND SYMPTOMS
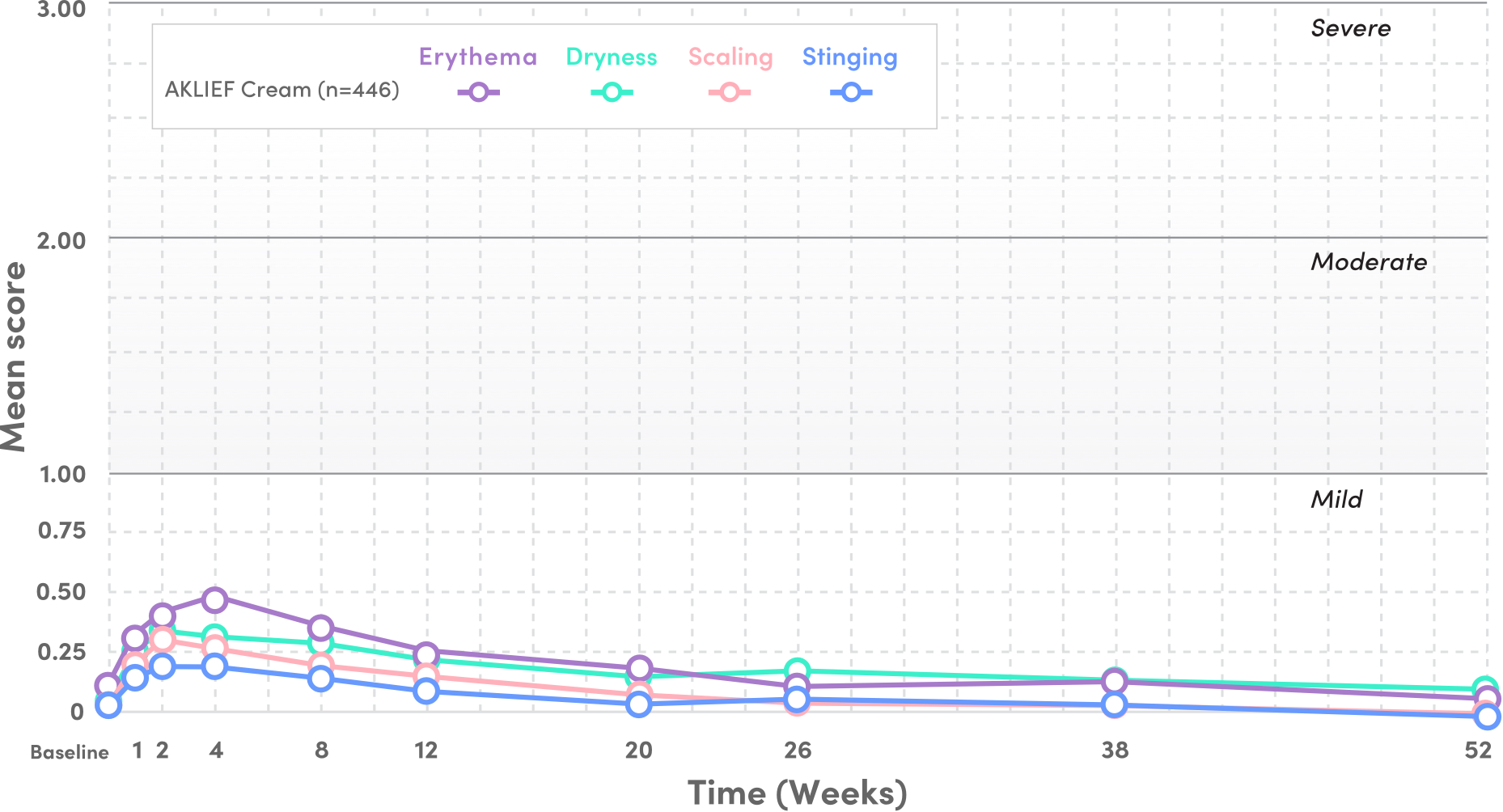
- Mean tolerability scores for erythema, scaling, dryness, and stinging/burning peaked at Week 1 on the face and decreased thereafter5
- Local tolerability signs and symptoms were evaluated on a 4-point severity scale: 0 (none), 1 (mild), 2 (moderate), and 3 (severe) from baseline to Week 52—all scores stayed below 1 through Week 525
Study Design
DUAL STUDY
A multicenter, randomized, double-blind Phase 4 clinical trial to evaluate the efficacy and safety of AKLIEF® (trifarotene) Cream, 0.005% with oral doxycycline once daily vs vehicle with doxycycline placebo in severe facial acne vulgaris.6
Study Design
202
PATIENTS COMBINED RANDOMIZED 2:1
from sites across US, Puerto Rico
Trifarotene topical acne daily; 120 mg enteric coated oral doxycycline (MPC) once daily6
AKLIEF Cream + Doxycycline
n=133
VEHICLE +
Placebo
n=69
PRIMARY ENDPOINT6
- Absolute change in total facial lesions from baseline to Week 12
SAFETY ENDPOINTS6
- Local tolerability (erythema, scaling, dryness, stinging/burning)
- Incidence of treatment-emergent adverse event
*The definitions of severity for the 5-point IGA (face) scale are: 0 (clear), 1 (almost clear), 2 (mild), 3 (moderate), and 4 (severe).6
THE DUAL STUDY SHOWED SIGNIFICANT RESULTS FOR PATIENTS WITH SEVERE ACNE.6
Phase 4 study of AKLIEF® (trifarotene) Cream, 0.005% with 120 mg enteric coated oral doxycycline in a population with severe acne.6
Significant Reduction in Total Facial Lesion Count at Week 12 vs Baseline6
67%†
REDUCTION IN TOTAL LESIONS FROM BASELINE AT WEEK 12
VS 45.5% REDUCTION WITH VEHICLE AND PLACEBO6
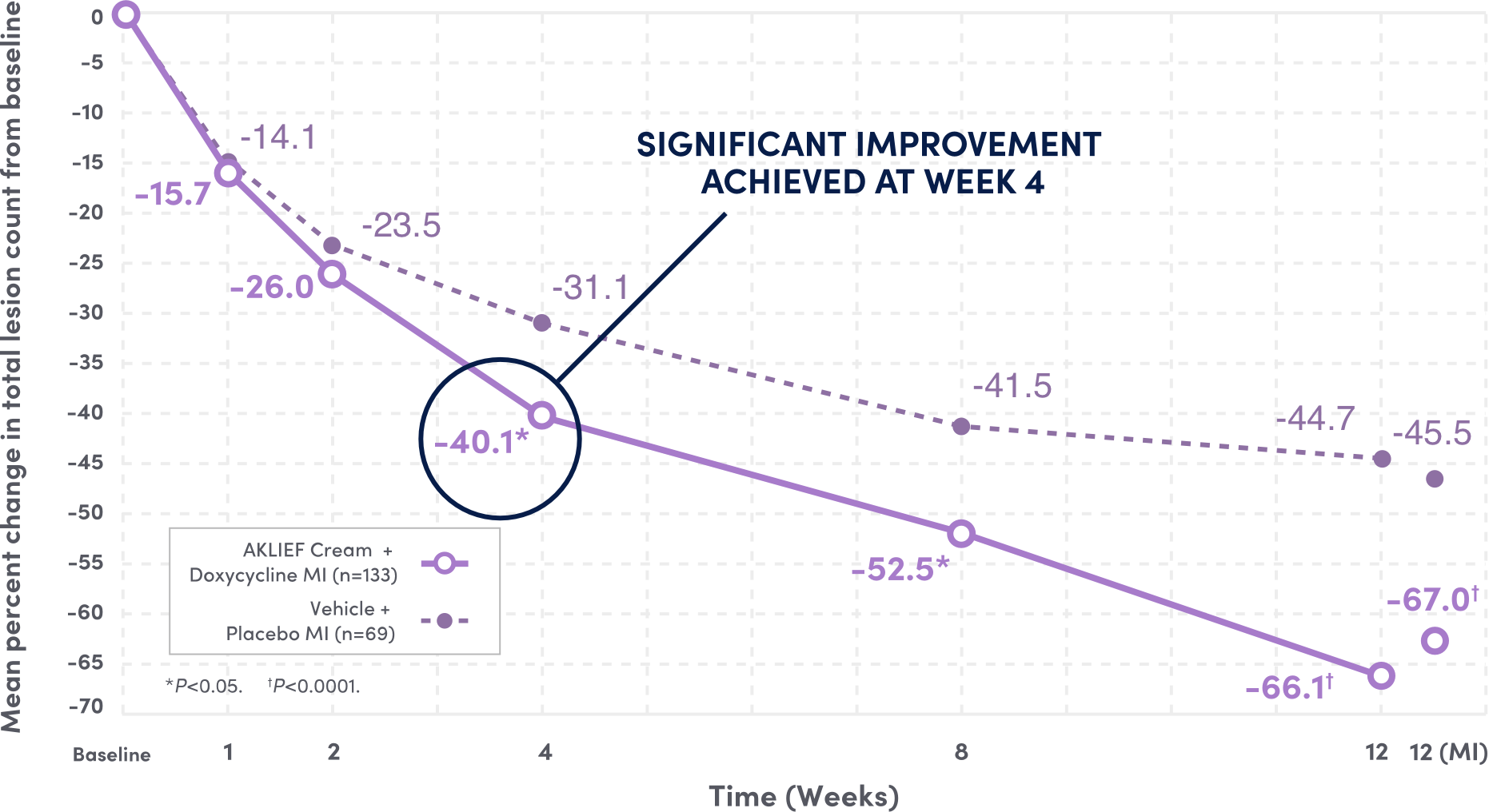
Figure is based on observed case analysis. Results of MI statistical analysis at Week 12 are included. For percent change in total lesion count from baseline (MI), the difference between treatment groups [95% CI] was -8.6 [-16.5, -0.7] at Week 4 (P=0.0334), -12.1 [-20.0, -4.2] at Week 8 (P=0.0028) and -21.5 [-28.9, -14.1] at Week 12 (P<0.0001).
CI=confidence interval; MI=multiple imputation statistical modeling.6
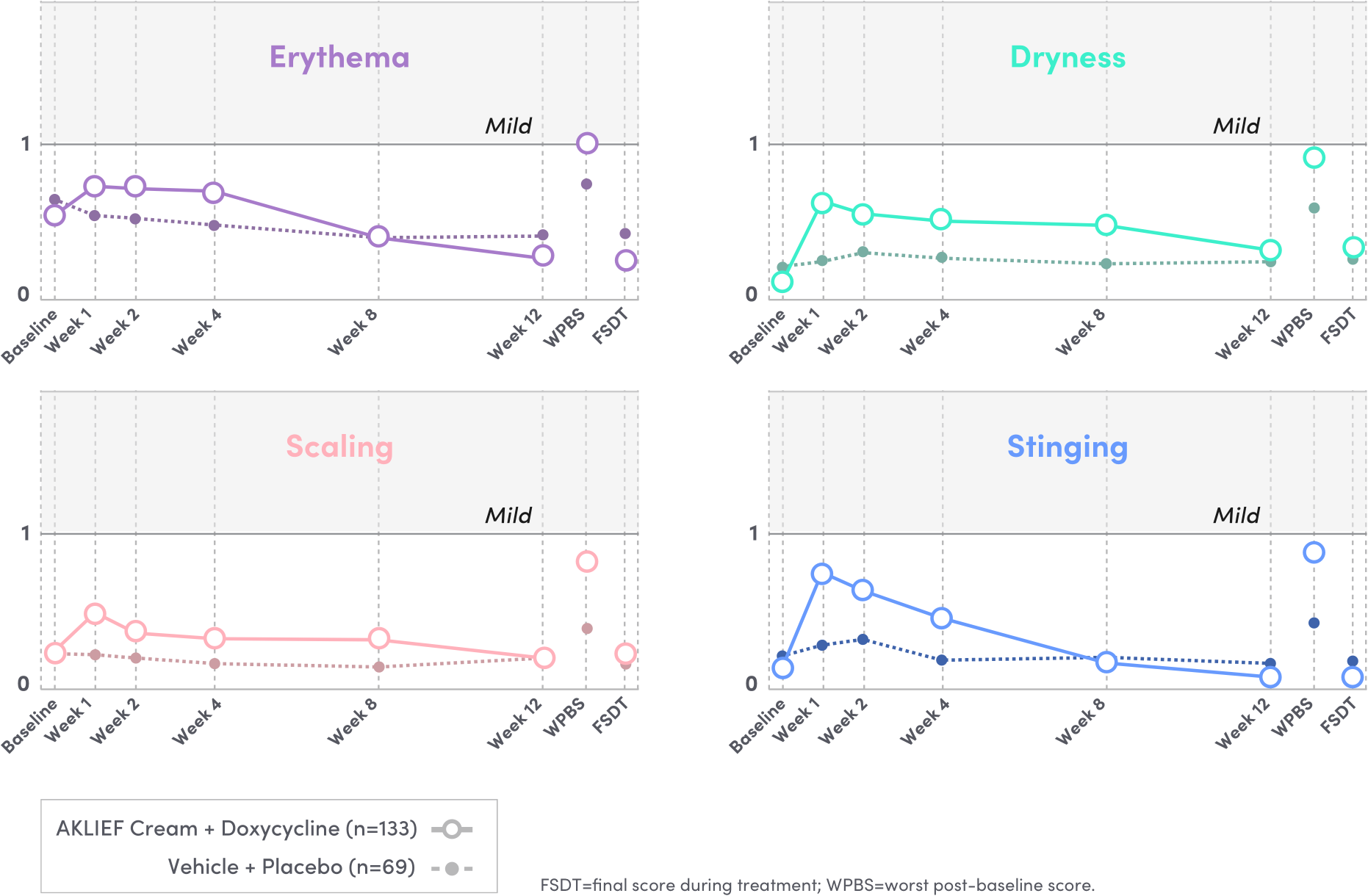
SAFE AND WELL-TOLERATED FOR PATIENTS WITH SEVERE ACNE.6
Phase 4 study of AKLIEF® (trifarotene) Cream, 0.005% with 120 mg enteric coated oral doxycycline in a population with severe acne.6
Local irritation was
MILD & TRANSIENT
AT WEEK 1 AND REDUCED THEREAFTER6
- The most common adverse reactions (incidence ≥ 1%) in patients treated with AKLIEF Cream were application site pruritus (itching) and sunburn6
- AKLIEF Cream and enteric coated oral doxycycline 120 mg was well tolerated, with tolerability comparable to placebo6
- The incidence of treatment-emergent adverse events was low6
SECONDARY ENDPOINTS6
- Investigator's Global Assessment (IGA)* facial success rate at Week 12, defined as BOTH IGA 0 (clear) or 1 (almost clear) AND ≥2-grade IGA improvement
- Percentage change in facial inflammatory and non-inflammatory lesions from baseline to Week 12
SAFETY ENDPOINTS6
- Local tolerability (erythema, scaling, dryness, stinging/burning)
- Incidence of treatment-emergent adverse event
*The definitions of severity for the 5-point IGA (face) scale is: 0 (clear), 1 (almost clear), 2 (mild), 3 (moderate), and 4 (severe).6
DOUBLE THE IGA SUCCESS RATE AT WEEK 12 VS VEHICLE.6
Phase 4 study of AKLIEF® (trifarotene) Cream, 0.005% with 120 mg enteric coated oral doxycycline in a population with severe acne.6
Proportion of Subjects Achieving IGA Success (ITT)6
ALTHOUGH 31.7% OF PATIENTS
ACHIEVED IGA SUCCESS,
78%
OF SUBJECTS OVERALL ACHIEVED AT LEAST A 2-GRADE IGA IMPROVEMENT, P<0.0001 (MI).6
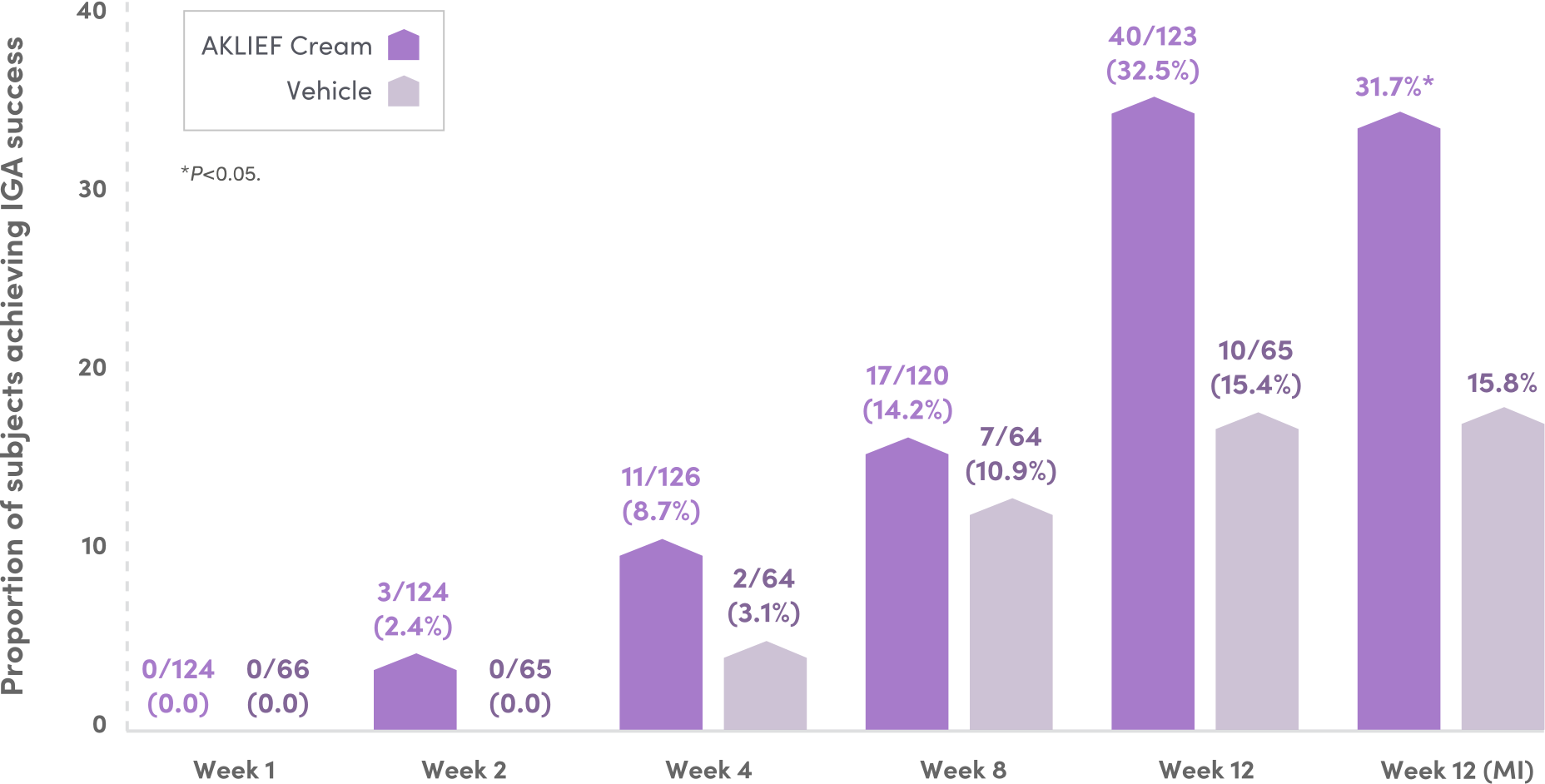
Figure is based on observed case analysis. Results of statistical analysis at Week 12 are included. IGA success is defined as IGA score 0 (clear) or 1 (almost clear), and at least a 2-grade improvement from baseline. (31.7 vs 15.8; P=0.0107; MI).
IGA=Investigator's Global Assessment; ITT=intent to treat; MI=multiple imputation.6

