Before & after photos
ACNE RELIEF YOUR PATIENTS CAN SEE.Results from the PIVOTAL studies.
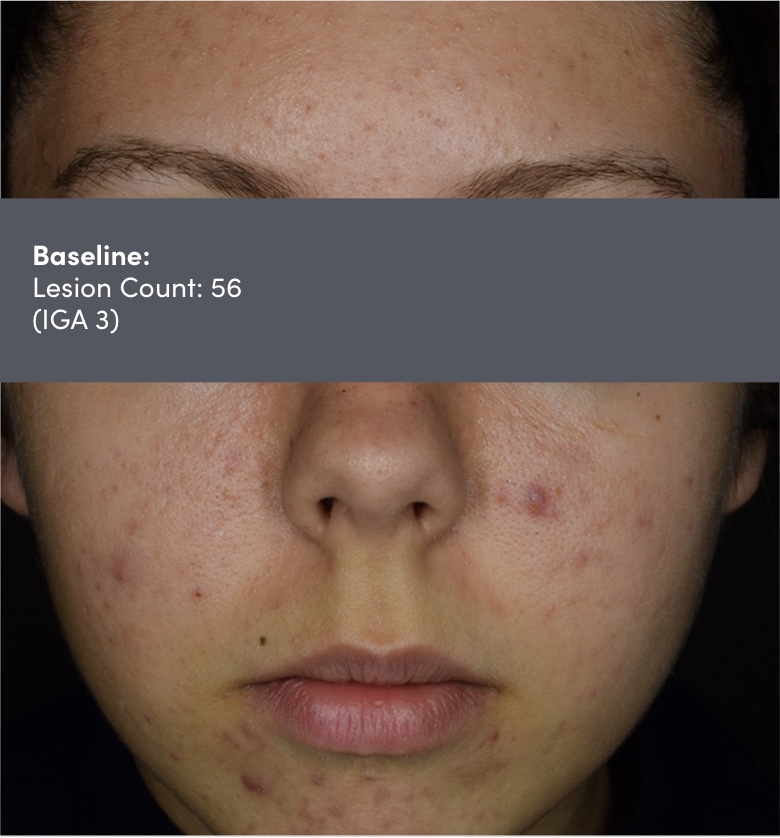
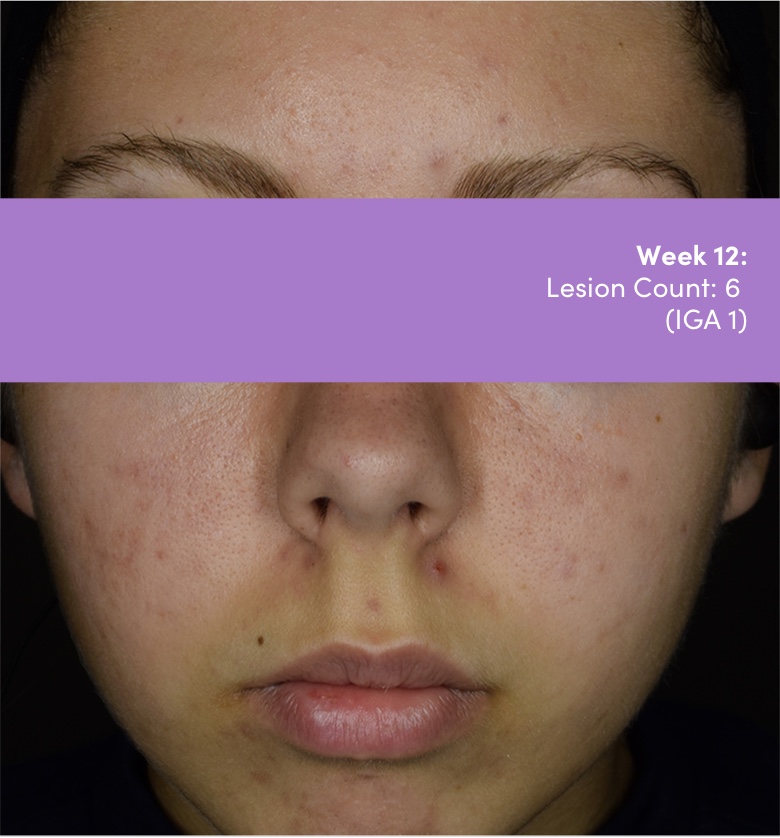
Study 18251: Double-blind, randomized, vehicle-controlled safety and efficacy 12-week trial of trifarotene cream vs vehicle cream.
IGA=Investigator's Global Assessment.
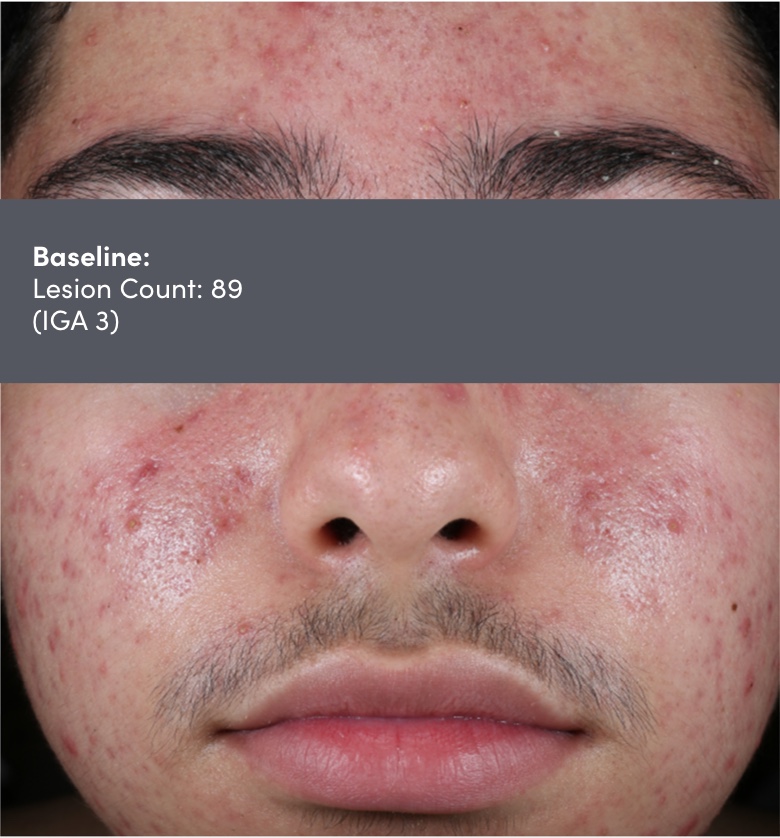
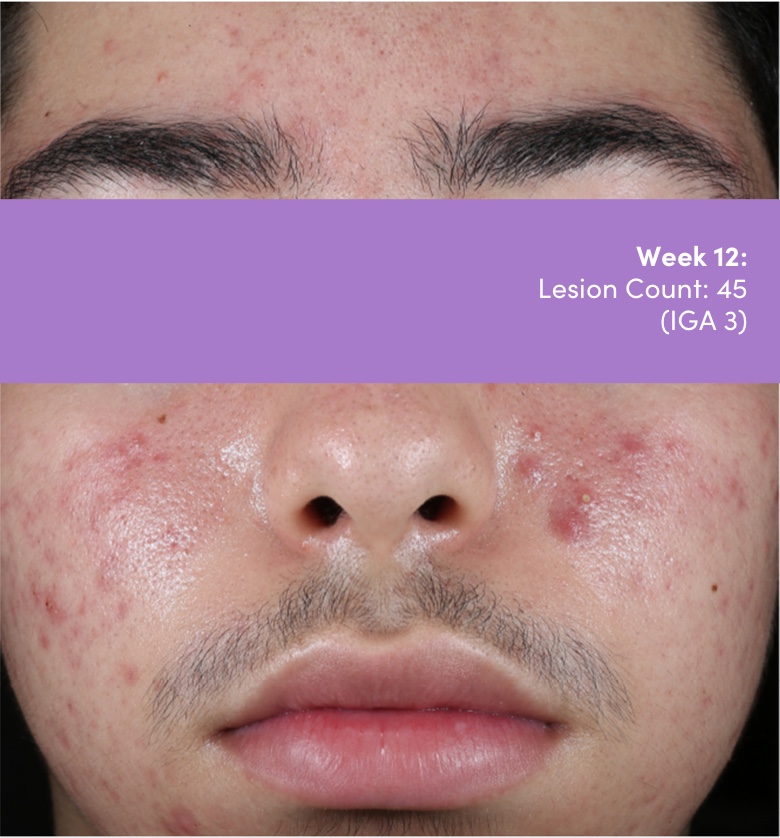
STUDY 118295: Open-label, single-arm study of trifarotene cream for 24 weeks.
IGA=Investigator's Global Assessment.
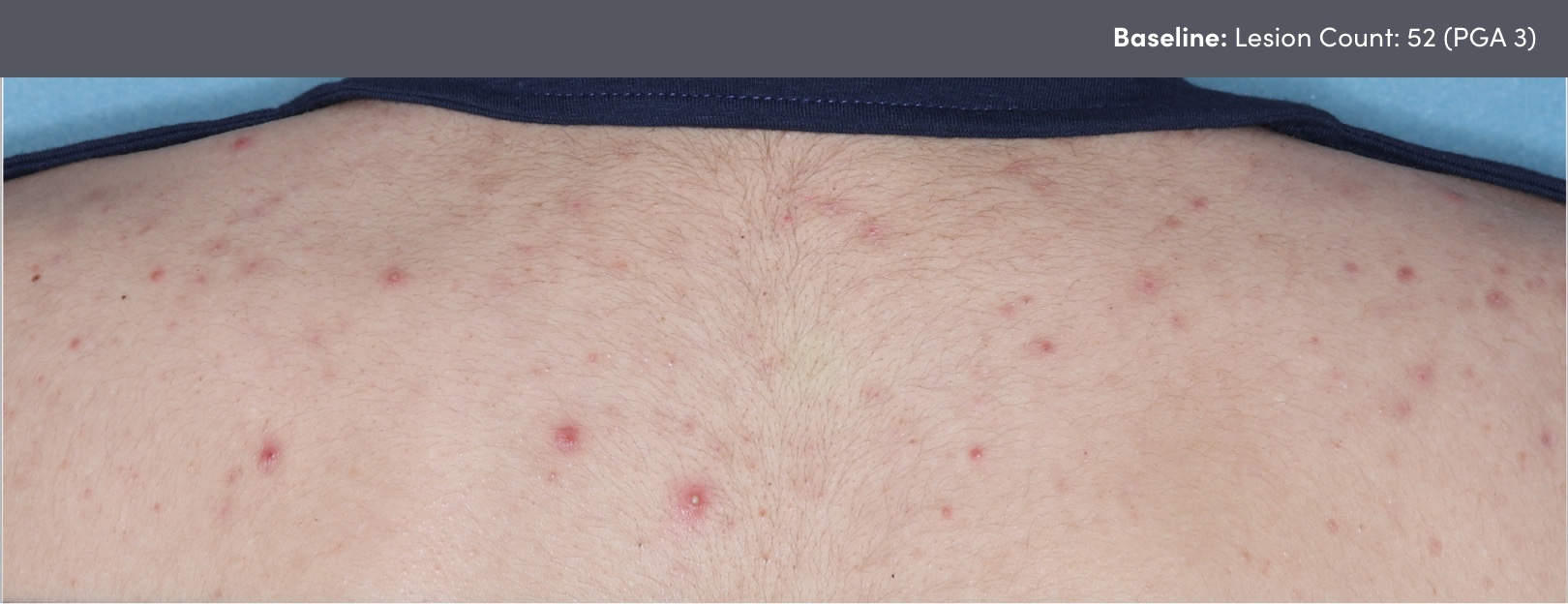
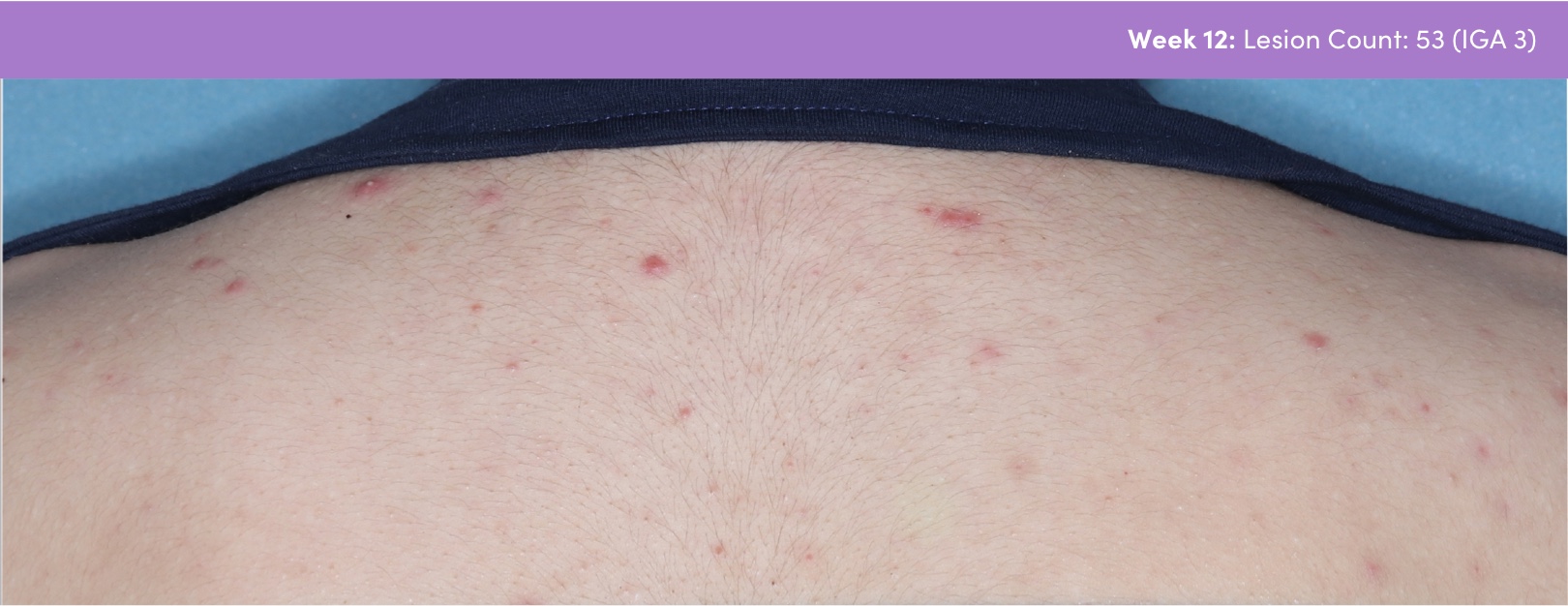
STUDY 118295: Open-label, single-arm study of trifarotene cream for 24 weeks.
PGA=Physician's Global Assessment.
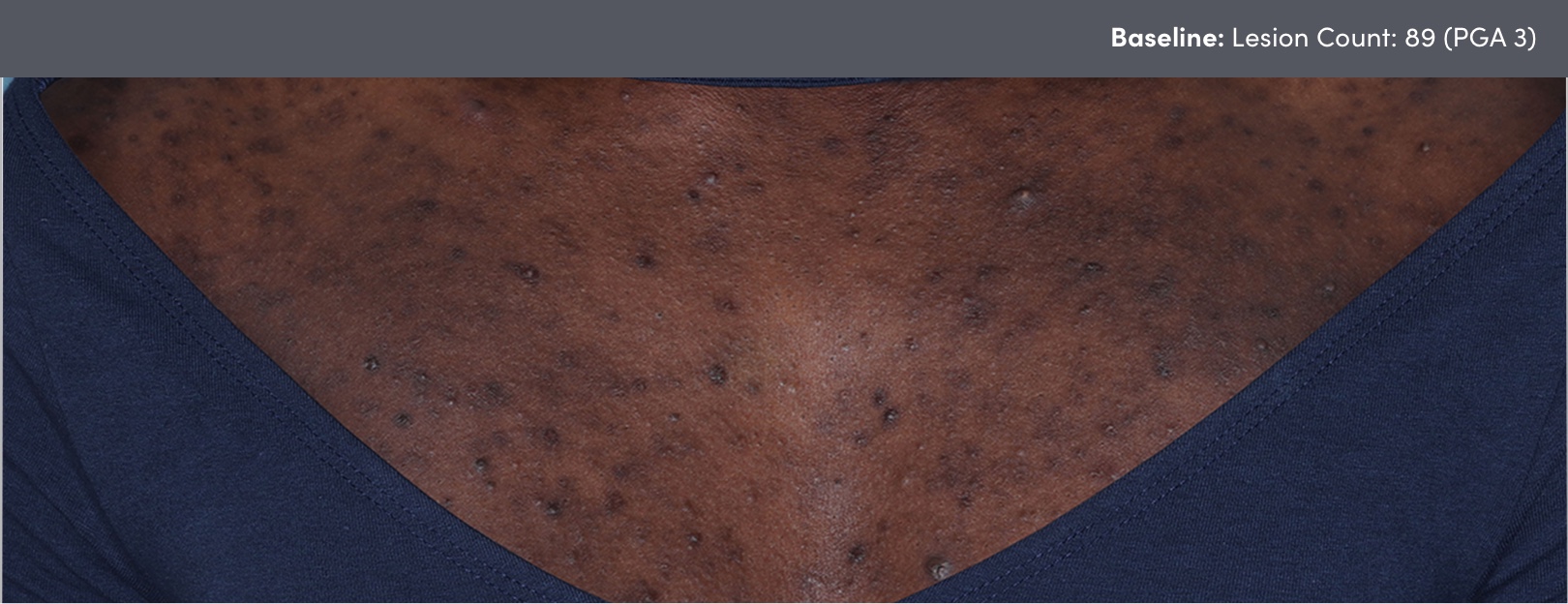
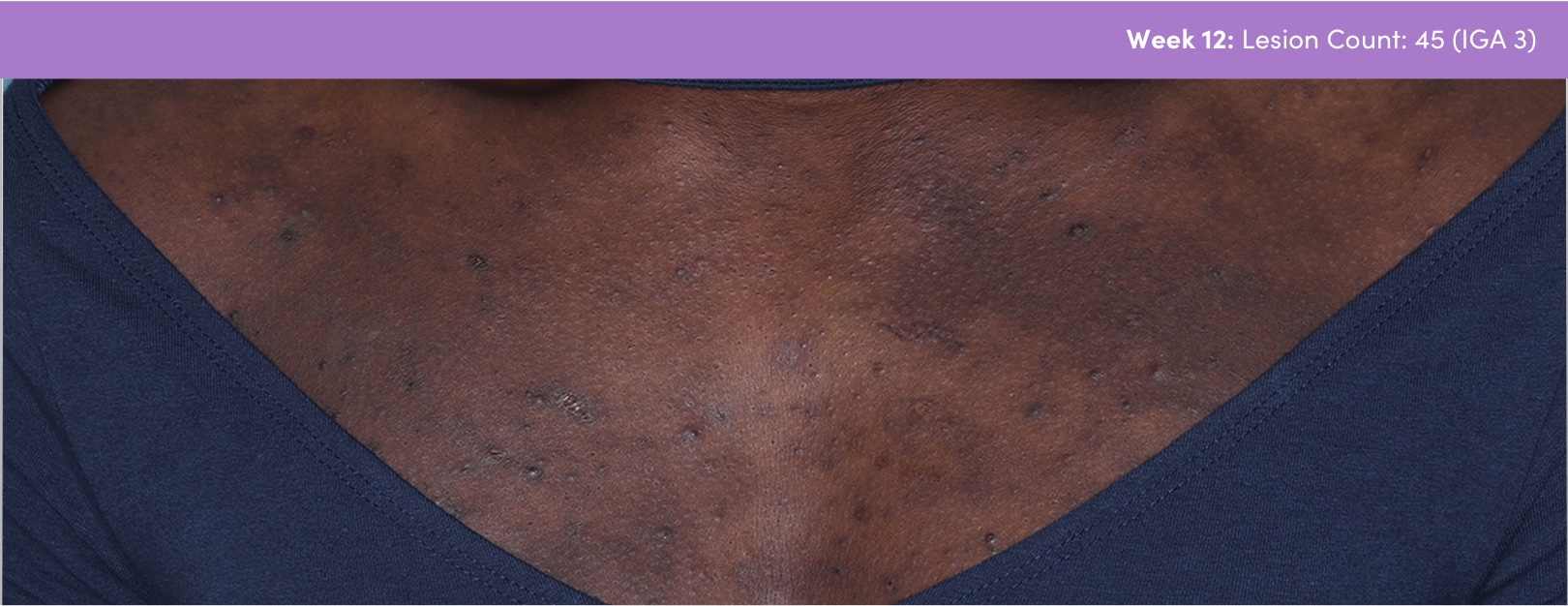
STUDY 118295: Open-label, single-arm study of trifarotene cream for 24 weeks.
PGA=Physician's Global Assessment.
Results from the DUAL study.
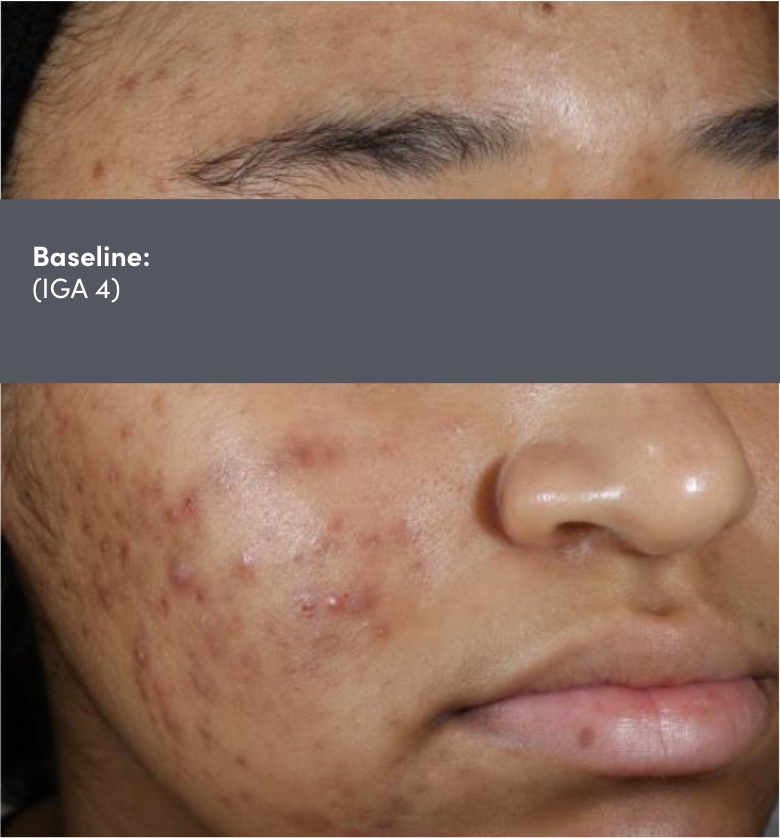
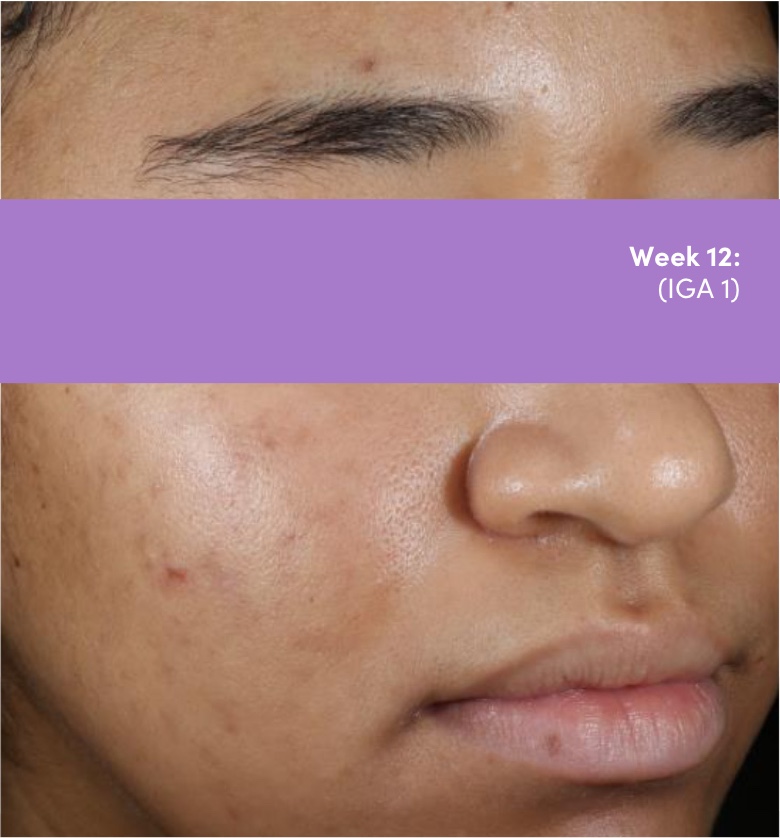
STUDY 118295: Phase 4 double-blind, randomized, placebo and vehicle-controlled safety and efficacy 12-week study of trifarotene cream and doxycycline vs vehicle cream and placebo.
IGA=Investigator's Global Assessment.
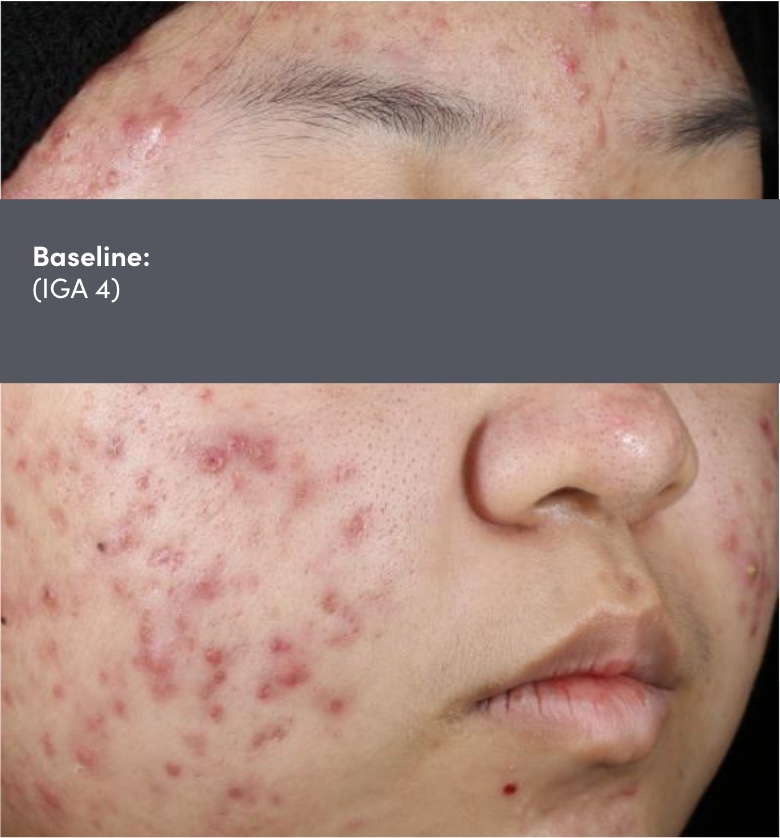
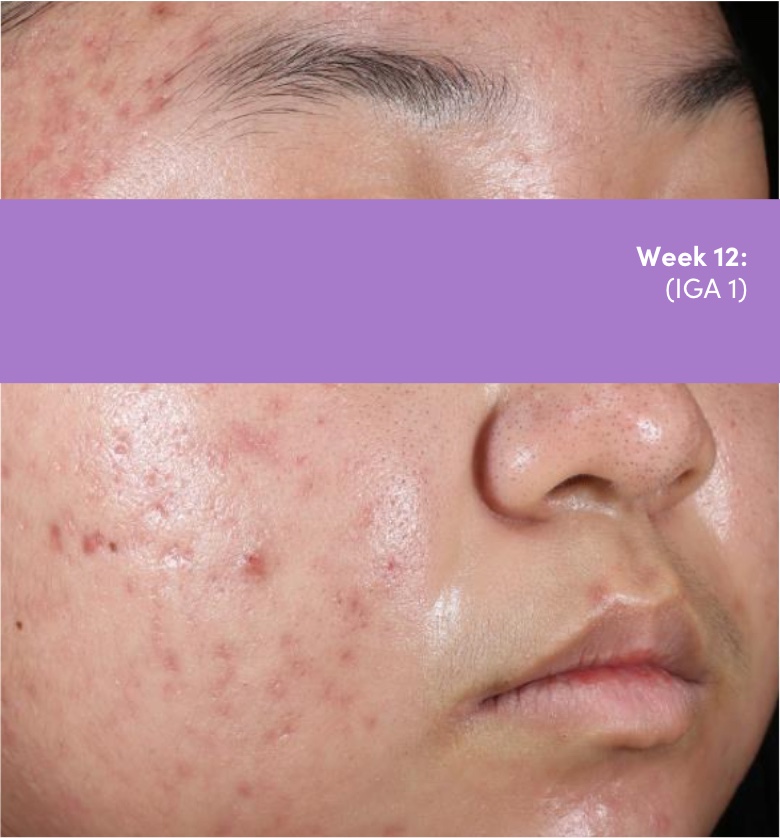
STUDY 118295: Phase 4 double-blind, randomized, placebo and vehicle-controlled safety and efficacy 12-week study of trifarotene cream and doxycycline vs vehicle cream and placebo.
IGA=Investigator's Global Assessment.

Treating acne doesn't have to break the bank.*
PATIENT SAVINGS CARD

